The Periodic Table – Key Highlights
Here’s a quick look at what we’ll cover in this guide:
- The Periodic Table of the elements is a master chart organising all 118 known chemical elements.
- Elements are arranged by their increasing atomic number, which represents the number of protons in an atom’s nucleus.
- The table’s layout reveals patterns in the properties of the elements, such as reactivity and electron configuration.
- Vertical columns are called groups of elements, which share similar chemical behaviors.
- Horizontal rows, known as periods, indicate the number of electron shells an element’s atoms possess.
Introduction
Have you ever wondered how scientists keep track of all the building blocks of matter? The answer is the Periodic Table of the elements, a foundational chart in chemistry. This powerful tool organises all known chemical elements in a logical and easy-to-understand way. It arranges them by their unique atomic number, helping us see patterns and relationships at a glance. Think of it as the ultimate cheat sheet for understanding everything from the air we breathe to the metals in our phones.
The Purpose and Importance of the Periodic Table
The Periodic Table is far more than just a colorful chart; it’s a vital tool for predicting how elements will behave and interact. The creator of the Periodic Table, Dmitri Mendeleev, first organised elements by their atomic mass in 1869, noticing that certain chemical properties reappeared periodically.
This simple observation revolutionised chemistry. Today, the modern Periodic Table is indispensable in both theoretical and applied chemistry, allowing scientists and students to understand relationships between elements without memorising every single fact. It’s the roadmap for all chemical exploration. Below, we’ll explore why chemists rely on it and how it helps predict element behaviors.
Why Every Chemist Uses the Periodic Table
For any chemist, the Periodic Table of the elements is a fundamental reference. Much like a world map for a geographer, it provides essential context for navigating the world of matter. Organisations like the American Chemical Society (ACS) recognise it as a cornerstone of chemical education and research. Its structure allows for a quick assessment of an element’s likely chemical properties just by looking at its location.
The table’s brilliance lies in its organisational power. When Dmitri Mendeleev first developed his version in the late 19th century, he even left gaps for elements that hadn’t been discovered yet, correctly predicting their properties. This predictive capability was a massive breakthrough and solidified the table’s importance.
Today, from students learning the basics to researchers developing new materials, everyone uses the table to understand relationships, predict reactions, and organise the vast amount of information about the elements. It remains the single most important document in chemistry.
How It Helps Predict Element Properties
One of the most powerful features of the Periodic Table is its ability to help predict the properties of the elements. By understanding an element’s position, you can make educated guesses about its behavior. For example, elements in the same column (group) tend to have a similar oxidation state, which describes how they might bond with other elements.
Initially, elements were sorted by atomic weight, but the modern table uses atomic number. This change refined the table’s predictive accuracy. Now, trends in properties like atomic radius, electronegativity, and ionisation energy can be clearly seen as you move across rows and down columns. Interactive Periodic Tables available online, like the one from PubChem, allow you to visualise these trends instantly. [1]
These patterns save chemists from having to perform endless theoretical calculations or experiments. Instead, they can use the table as a starting point to hypothesise how a substance might react, what its melting point could be, or whether it will conduct electricity, all based on established periodic trends.
How Elements Are Organised on the Periodic Table
The organisation of the Periodic Table is what makes it so useful. Elements are arranged in order of increasing atomic number, starting with hydrogen at 1 and moving from left to right across the chart. This layout isn’t random; it’s structured into rows and columns that reveal deep connections between the elements.
The horizontal rows are called periods, and the vertical columns are known as groups of elements. The table is also divided into sections called blocks. An element’s position provides a wealth of information about its atomic structure and chemical behavior. We will now look closer at these structural components and what they tell us.
Periodic Table of elements with names and symbols
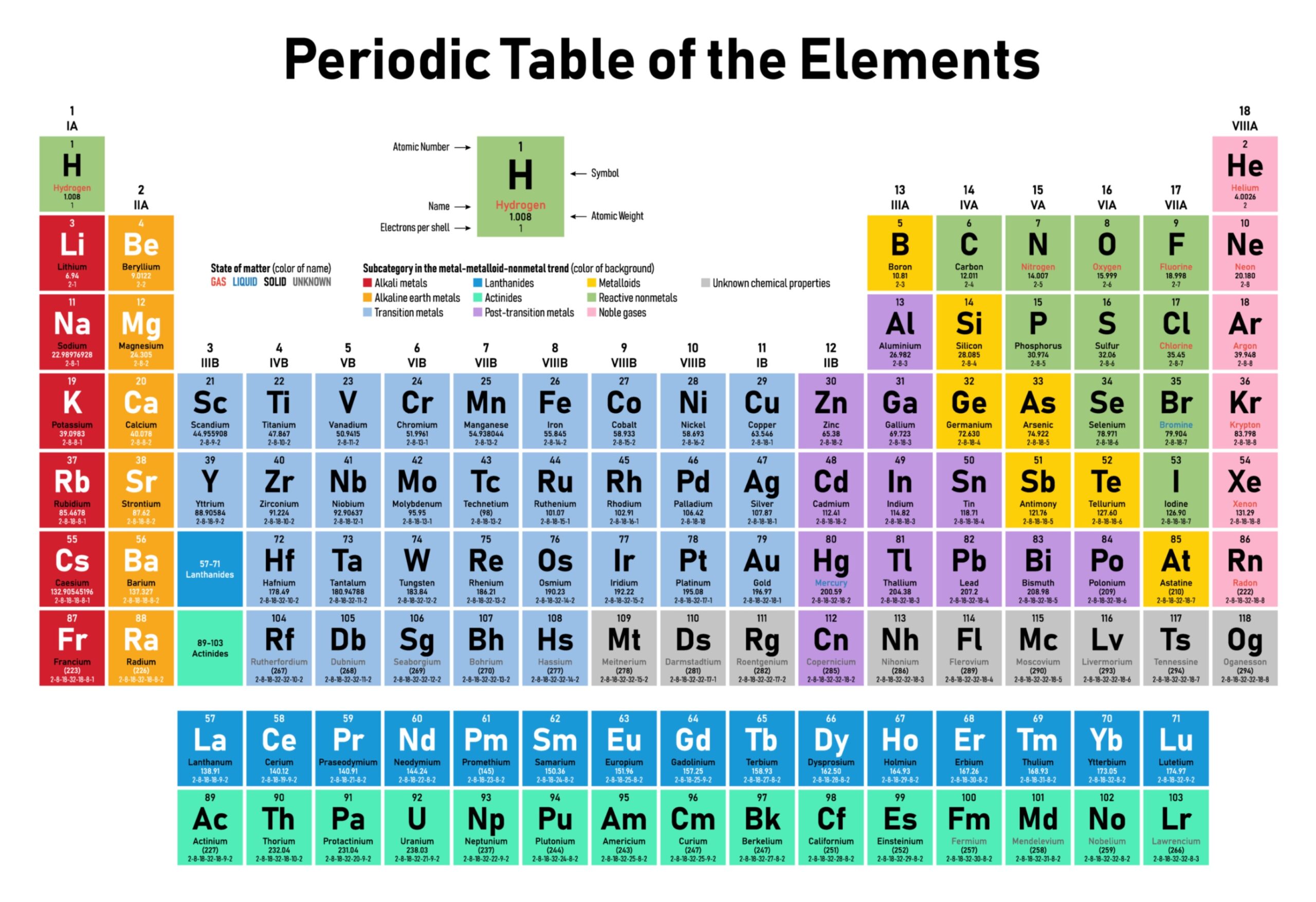
Layout: Groups, Periods, and Blocks Explained
Understanding the layout of the Periodic Table starts with its main components: periods, groups, and blocks. The horizontal rows are called periods. All elements within the same period have the same number of electron shells, which are the orbits electrons follow around the atom’s nucleus. As you move across a period, elements gain one proton and one electron.
The vertical columns are the groups of elements, numbered 1 through 18. Elements in the same group share similar chemical properties because they have the same number of electrons in their outermost shell. This is why elements in a group often react in similar ways. Some key groups have special names:
- Group 1: The alkali metal elements (except hydrogen) are highly reactive.
- Group 2: Alkaline earth metal elements, reactive but less than the alkali metal elements.
- Groups 3-12: These are known as the transition metal elements.
- Group 17: Halogens, highly reactive non-metals
- Group 18: The noble gases are very non-reactive.
Finally, the table is divided into s, p, d, and f blocks. These blocks correspond to the type of atomic orbital the outermost electrons occupy, which further helps classify and predict an element’s properties.
Elements in Periodic Table – Their Position and What It Reveals About Reactivity
An element’s position on the Periodic Table is a direct clue to its reactivity. Generally, reactivity is highest for metals on the far left (like sodium) and nonmetals on the upper right (like fluorine), while the elements in the middle and on the far right are less reactive. This trend is closely linked to an element’s atomic structure.
For example, the element position dictates its atomic radius, or the size of its atoms. As you move down a group, the atomic radius increases, making it easier for the atom to lose an outer electron and react. Conversely, moving across a period from left to right, atoms hold their electrons more tightly, influencing how they bond.
The most stable elements are the noble gas group on the far right. Their outer electron shells are full, making them very reluctant to react with anything. This stability is a goal for other elements, which react to achieve a similar electron configuration. The concept of a stable isotope, which doesn’t undergo radioactive decay, is another layer of stability that varies by element.
Decoding Symbols and Numbers on the Periodic Table of Elements
Each square on the Periodic Table contains key information about an element, presented in a shorthand format. You’ll typically see an element symbol, which is a one- or two-letter abbreviation, along with a few important numbers. These pieces of data are the foundation for understanding the element’s identity.
The most prominent numbers are the atomic number and the atomic mass. Together with the symbol, they provide a snapshot of the element’s core properties and its place in the universe of chemistry. Let’s break down what each of these components means.
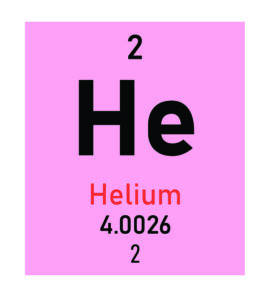
What Each Element Symbol Represents
Every element is assigned unique names and symbols that are used universally by scientists. The symbol is a one- or two-letter abbreviation derived from the element’s name, which can be in English, Latin, or another language. For example, the first element, hydrogen, has the symbol ‘H,’ while gold has the symbol ‘Au’ from its Latin name, aurum.
These symbols are essential for writing chemical formulas and equations. They provide a simple way to represent the 118 known chemical elements without having to write out their full names each time. While many substances we encounter are compounds, a few elements, like gold and carbon, can be found in their pure form in nature.
The naming of new elements is overseen by the International Union of Pure and Applied Chemistry (IUPAC). Here are a few examples of elements and their symbols:
|
Element Name |
Symbol |
|
Hydrogen |
H |
|
Helium |
He |
|
Carbon |
C |
|
Oxygen |
O |
|
Gold |
Au |
Meaning of Atomic Number and Mass
The atomic number, usually found at the top of an element’s box, is the most important identifier. It represents the number of protons in the nucleus of a single atom of that element. Since every element has a unique number of protons, the atomic number defines the element. For instance, any atom with 6 protons is a carbon atom.
In a neutral atom, the atomic number also equals the number of electrons orbiting the nucleus. This balance is crucial for understanding an element’s chemical bonding behavior. The Periodic Table is arranged in order of increasing atomic number, which creates the periodic patterns of properties.
The other key number is the atomic mass. This value is the weighted average mass of all the natural isotopes of an element. For elements with no stable isotopes, the mass number of the most stable isotope is often listed instead. This number reflects the total count of protons and neutrons in the nucleus.
Exploring Element Groups and Their Similar Behaviors
One of the most fascinating aspects of the Periodic Table is how it organises elements with similar properties into vertical columns. These element groups are like families, where each member shares key characteristics with the others. This is because they all have the same number of electrons in their outermost shell.
These shared chemical traits mean that elements in the same group often react in predictable ways and form compounds with a similar oxidation state. Exploring these groups helps simplify the study of chemistry from 118 individual elements to a handful of related families. Let’s examine some of these groups and their common behaviors.
Alkali, Alkaline Earth, Transition, and Other Groups
The Periodic Table’s 18 groups are home to several well-known element families, each with its own distinct identity. The elements in Group 1 are the highly reactive alkali metal family (with the exception of hydrogen). Just next door in Group 2 are the alkaline earth metal elements, which are also reactive but less so than their neighbors.
Spanning the center of the table from Groups 3 to 12 is the large block of transition metal elements. This group includes familiar metals like iron, copper, and gold, which are known for their strength and ability to form colorful compounds.
Other important groups include the halogens in Group 17 and the noble gases in Group 18. Additionally, two rows are often shown separately at the bottom of the table:
- The lanthanides and actinides are known as the inner transition metals.
- Many of these are considered rare earth elements, with the actinides being radioactive.
Shared Chemical Traits Within Groups
Elements within the same group exhibit similar properties because they share the same number of valence electrons—the electrons in the outermost shell. These electrons are the primary drivers of an element’s chemical behavior, as they are the ones involved in forming bonds with other atoms. For example, every element in Group 1 has one valence electron, which it tends to lose easily, making these elements highly reactive.
These shared electronic structures lead to predictable patterns in chemical properties. Elements in a group often have the same common oxidation state, meaning they tend to gain, lose, or share the same number of electrons when forming compounds. This is why sodium (Na) and potassium (K), both in Group 1, form similar compounds like NaCl and KCl.
While their physical properties, such as melting point and density, may change as you move down a group, their fundamental chemical nature remains consistent. This principle of recurring properties is the essence of the periodic law and makes the table an incredibly powerful predictive tool.
Conclusion
In summary, the Periodic Table is not just a collection of elements; it’s a fundamental tool that provides insight into the behavior and relationships of different substances. Understanding its layout, symbols, and groups can enhance your ability to predict chemical reactions and comprehend the intricate world of chemistry. By recognising the significance of element organisation and the properties they share, you can develop a deeper appreciation for this scientific marvel. If you’re eager to dive deeper into the world of chemistry, don’t hesitate to seek out more resources or expert guidance to enrich your knowledge further. Happy exploring!
Frequently Asked Questions
Where can I download a printable Periodic Table?
You can find high-quality, printable versions of the Periodic Table of the elements on various scientific websites. Organisations like the American Chemical Society (ACS) and public databases such as PubChemoffer free, downloadable PDFs that often include details like the most stable isotope for each element.
What are some tips for memorising the elements and their symbols?
For memorising elements, try using mnemonic devices or songs to remember them in order of atomic number. Flashcards are a classic tool for matching an element symbol to its name. Many websites also offer interactive games that make learning the table fun and engaging.
How has the Periodic Table changed as new elements were discovered?
The Periodic Table is a living document that grows as a new element is discovered. The International Union of Pure and Applied Chemistry (IUPAC) is responsible for validating discoveries, often of synthetic elements created via radioactive decay, and officially naming them. [2] The table has expanded significantly since the first element was identified.
Citations: [1] PubChem. “Periodic Table of Elements.” National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/ptable/ [2] “About.” International Union of Pure and Applied Chemistry. https://iupac.org/about/