Periodic Table Group 2 – Key Highlights
- Alkaline earth metals are the six elements found in Group 2 of the Periodic Table.
- They share similar physical properties, including being shiny, silvery-white metals.
- Their chemical properties are defined by having two valence electrons, making them quite reactive.
- The atomic radius increases as you move down the group, affecting their reactivity.
- These metals can withstand high temperatures and form many useful calcium compounds.
- Calcium is the most common element of the group and is vital for life.
Introduction
Welcome to the world of alkaline earth metals! These fascinating elements occupy the second column of the Periodic Table. If you’ve ever heard of the highly reactive alkali metals in Group 1, you can think of these as their slightly calmer cousins. This group includes some familiar names and plays a huge role in everything from our bodies to major industries. Let’s explore what makes these six metals so special, from their atomic structure to their everyday uses.
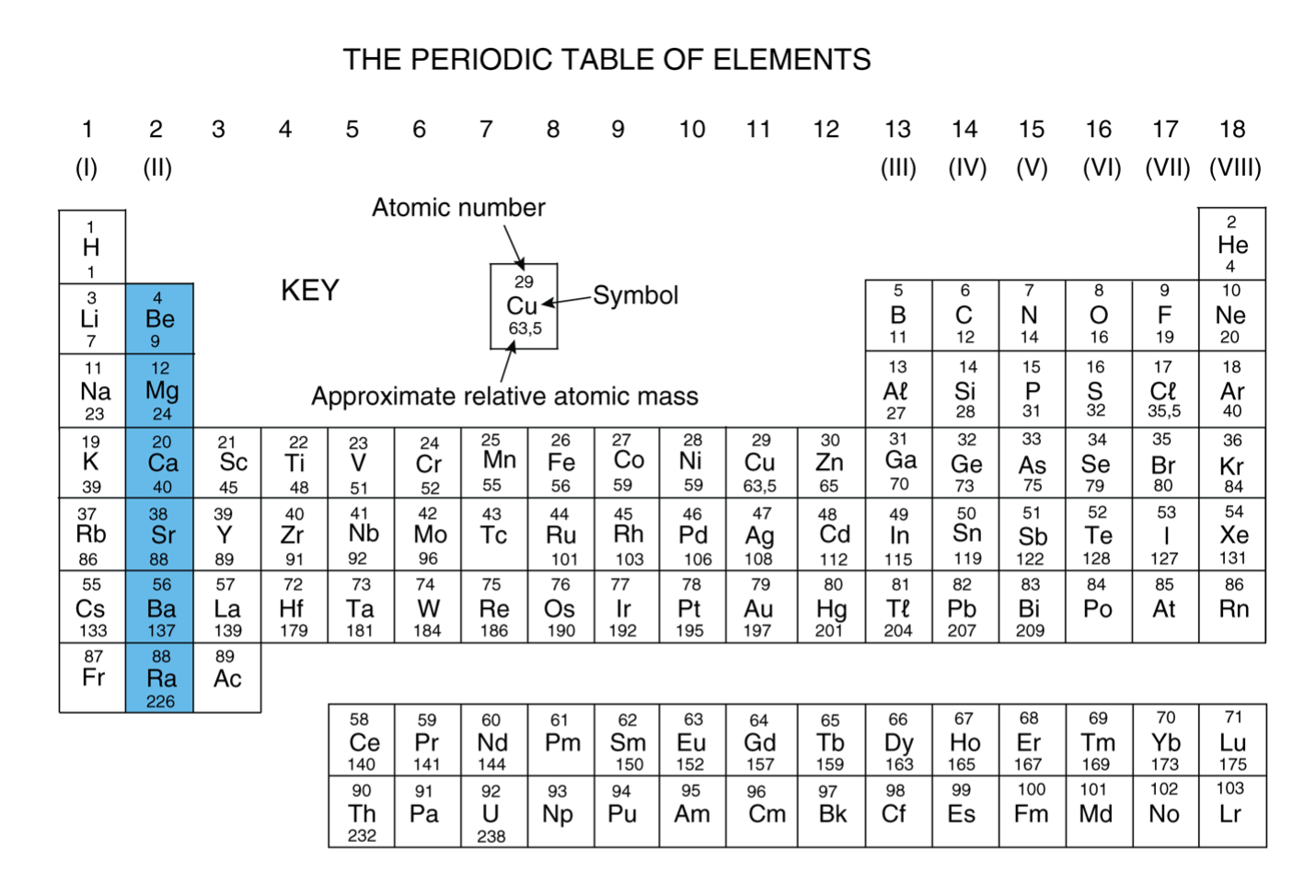
Overview of Alkaline Earth Metals in the Periodic Table
The alkaline earth metals are a family of elements grouped together in the Periodic Table for good reason. From beryllium to radium, they share a common electronic configuration that dictates much of their behavior. As you move down the group, the atomic number increases, and you’ll notice clear trends in their properties.
These elements are found in the Earth’s crust, but never in their pure form because they are too reactive. Understanding their position and basic structure is the first step to appreciating their unique chemistry. Next, we will cover which elements are in this group and why they earned their distinct name.

Elements Included in Group 2
The elements that make up Group 2 are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Each one has its own unique characteristics, though they share family traits. For example, beryllium is the first member of the alkaline earth metals and is known for being very light and strong, forming unique beryllium compounds.
Further down the group, you find magnesium and calcium, which are essential to life. Calcium salts, for instance, are the building blocks of our bones and teeth. Strontium and barium are less common but have important industrial uses, such as in the compound barium sulfate, which is used in medical imaging.
Finally, radium is the heaviest element in the group and is highly radioactive. All isotopes of radium are unstable, meaning they decay over time. This radioactivity makes it different from the other members, which all have stable isotopes.
Why They Are Called Alkaline Earth Metals
Have you ever wondered where the name “alkaline earth metals” comes from? The name is a nod to the properties observed by early chemists. The “alkaline” part comes from the fact that their oxides, like calcium oxide, react with water to form basic, or alkaline, solutions. For example, when calcium oxide mixes with water, it produces calcium hydroxide.
The “earth” part of the name is an old term for nonmetallic substances that are insoluble in water and stable at high temperatures. Early chemists noted that compounds like beryllium oxide and the mineral barite (a source of barium) didn’t break down when heated.
Combining these two characteristics gives us the name for this group. They are metals whose “earths” (oxides) produce alkaline solutions. This simple name neatly summarises a key chemical trait of the entire family.
Position and Organisation in the Table
You can find the alkaline earth metals located in Group 2 of the Periodic Table, right next to the alkali metals. This specific column placement is determined by their atomic structure. Every element in this group has two outermost electrons in its s-orbital. This shared feature is what unites them and dictates their chemical behavior.
Because of their tendency to react, you will not find these elements in their pure form in nature. They are always bonded with other elements. Their position in Group 2 means they have a higher atomic number than the Group 1 element in the same row but a lower one than the elements to their right.
Here’s a quick look at their atomic numbers and electron configurations:
Element | Atomic Number | Electron Configuration |
Beryllium | 4 | [He]2s² |
Magnesium | 12 | [Ne]3s² |
Calcium | 20 | [Ar]4s² |
Strontium | 38 | [Kr]5s² |
Barium | 56 | [Xe]6s² |
Radium | 88 | [Rn]7s² |
Atomic Structure and Physical Characteristics
The atomic structure of the alkaline earth metals is the key to understanding their physical properties. With two valence electrons, these elements form stronger metallic bonds than their Group 1 neighbours. This results in them being harder, denser, and having a higher melting point.
As you move down the group, the atomic radius increases, which influences many of their characteristics. We will now look more closely at some of these physical traits, including their atomic radii, melting and boiling points, and their appearance.
Atomic and Ionic Radii
The atomic radius of the alkaline earth metals increases as you go down the group. This happens because each element adds a new electron shell. Despite this, their atoms are smaller than the alkali metals in the same period because of a higher nuclear charge pulling the electrons in more tightly.
When these elements react, their electronic configuration changes. They lose their two outermost electrons, forming positive ions with a +2 charge. These ions have a much smaller size than their original neutral atoms. The ionic radii also increase as you move down the group, following the same trend as the atomic radius.
The small size of the ions, especially at the top of the group, results in a high charge density. This property is important as it influences how these ions interact with other substances, like water. It takes significant energy, known as the second ionisation energy, to remove that second electron, but the resulting stability makes it worthwhile.
Melting and Boiling Points
One of the defining features of alkaline earth metals is their relatively high melting point and boiling point compared to alkali metals. This is because they have two valence electrons, which participate in creating stronger metallic bonds within the crystal structure. These stronger bonds require more energy to break, leading to higher temperatures needed for melting and boiling.
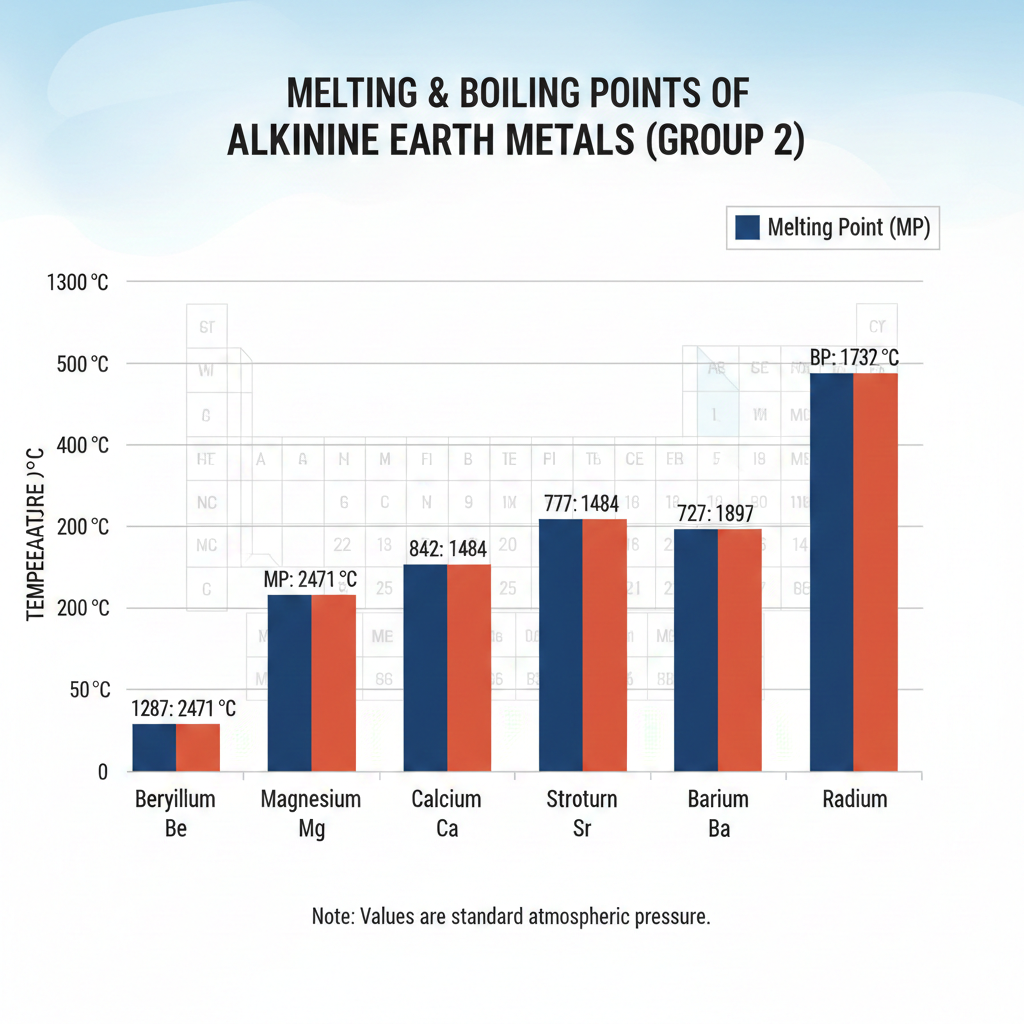
All alkaline earth metals are solids at room temperature. As you move down the group from beryllium to barium, the melting and boiling points generally decrease, with magnesium being a slight exception to this trend. This is because the atoms get larger, and the metallic bonds become slightly weaker over the greater distance.
Despite this downward trend, their ability to withstand high temperatures makes them useful in various applications where heat resistance is crucial. Beryllium, at the top of the group, has the highest melting point of them all.
Density and Appearance Comparisons
When you look at the alkaline earth metals, one of the first physical properties you’d notice is their appearance. In pure form, they are all a shiny, silvery white metal. This bright, metallic luster gives them a classic silver appearance, although they tarnish quickly when exposed to air.
In terms of density, these metals are denser than the alkali metals. This is due to their smaller atomic size and stronger metallic bonding, which packs the atoms more tightly together. The general trend for density is that it increases as you move down the group from magnesium to radium.
Interestingly, calcium is an exception to this trend, as it has the lowest density of the group. Beryllium is also notable for being very lightweight yet strong. These variations in density are just one example of the subtle differences that make each element in the group unique.
Chemical Properties of Alkaline Earth Metals
The chemical properties of alkaline earth metals are largely driven by their desire to achieve a stable electron configuration. They readily lose their two outer electrons, which defines their common oxidation state of +2. This makes them good reducing agents, meaning they donate electrons in chemical reactions.
Because they are so willing to react, you won’t find them in their pure form in nature. The reactions of group 2 elements with other substances reveal a clear pattern of reactivity, which we will explore next, along with how they form ions and what happens when they encounter water.
Reactivity Trends Within Group 2
The chemical reactivity of the alkaline earth metals increases as you move down the group. Beryllium is the least reactive, while barium is much more reactive. This trend is directly linked to how easily each element can give up its valence electrons.
This increasing reactivity is explained by a property called first ionisation energy, which is the energy needed to remove one electron. This energy decreases down the group because the outermost electrons are farther from the nucleus and are shielded by inner electron shells. This makes them easier to remove.
As a result, elements lower in the group react more vigorously. For example, the formation of an oxide layer on the metal’s surface happens much more readily with barium than with beryllium. This predictable increase in reactivity is a hallmark of the group’s chemical behavior.
Formation of Divalent Positive Ions
A key chemical trait of all alkaline earth metals is their tendency to form divalent ions, which are positive ions with a +2 charge. This happens because each element has two electrons in its valence shell. By losing these two outermost electrons, the atom achieves the stable electron configuration of the nearest noble gas.
This process defines their most common oxidation state, which is +2. Although it requires energy to remove two electrons, the resulting ion is so stable that the overall process is favorable in most chemical reactions. This consistent formation of +2 ions is what allows these metals to form predictable ionic compounds.
Except for the radioactive radium, all the elements in this group have stable isotopes that exhibit this same behavior. The drive to empty their valence shell and form these divalent ions is the fundamental reason for their reactivity and the types of bonds they create.
Reactions with Water and Other Compounds
The reaction of alkaline earth metals with water showcases their increasing reactivity down the group. Beryllium is unique in that it does not react with water, even at high temperatures. This is because it quickly develops a protective layer that prevents a reaction.
Magnesium reacts, but only with hot water or steam, to form magnesium hydroxide and hydrogen gas. In contrast, calcium, strontium, and barium are reactive enough to react with cold water. These reactions produce the corresponding metal hydroxide, such as calcium hydroxide, and liberate hydrogen gas.
These elements also react with other substances. For example, most will burn in oxygen to cause the formation of an oxide. Beryllium compounds often show more covalent character compared to the more ionic compounds of the other group members. This variety in reactions makes the group chemically diverse.
Comparing Group 2 (Alkaline Earth Metals) to Group 1 Elements
While they are neighbours on the Periodic Table, the alkaline earth metals have noticeable differences from the alkali metals in Group 1. Although both groups are reactive metals, their chemical properties and physical traits set them apart. These differences are rooted in their atomic structure—Group 2 elements have two valence electrons, while Group 1 has only one.
This extra electron affects everything from their atomic radius and reactivity to the types of compounds they form. Let’s take a closer look at these distinctions in reactivity, physical characteristics, and compound formation.
Differences in Chemical Reactivity
When it comes to chemical reactivity, the alkali metals are the clear winners. Group 1 elements are more reactive than the Group 2 elements in the same period. The main reason for this is that alkali metals only need to lose one electron to achieve a stable state, which requires less energy.
Alkaline earth metals, on the other hand, must lose two electrons. While their final +2 oxidation state is very stable, the energy required to remove two electrons is significantly higher than that needed to remove one. This makes them reactive, but not as intensely as their Group 1 neighbours.
This difference is evident in their reactions. For instance, the formation of an oxide happens more violently with alkali metals. The higher ionisation energies of the Group 2 elements serve to temper their reactivity in comparison.
Distinctive Physical Properties
The physical properties of alkaline earth metals are also quite distinct from alkali metals. Generally, Group 2 elements are harder, denser, and have a higher melting point. This is a direct result of having two valence electrons instead of one.
These two electrons per atom contribute to stronger metallic bonds. A stronger bond means more energy is required to pull the atoms apart, leading to solids that can withstand high temperatures better than alkali metals. This enhanced bonding also packs the atoms closer together, increasing their density.
While the atomic radius of a Group 2 element is smaller than its Group 1 neighbor in the same period, the stronger forces holding the metal together give it more robust physical properties. These traits make them more suitable for structural applications where strength and durability are important.
Compound Formation Variations
The variation in compound formation between Group 1 and Group 2 is all about the charge. Alkali metals form ions with a +1 charge, while alkaline earth metals form ions with a +2 charge. This difference in charge leads to different chemical formulas and properties for their compounds.
For example, Group 2 elements form compounds like calcium carbonate (CaCO₃) and beryllium oxide (BeO). In these, the metal ion has a +2 charge, balancing the negative charge of the other part of the molecule. This is different from a Group 1 compound like sodium chloride (NaCl), where sodium has a +1 charge.
This +2 charge leads to stronger ionic bonds in the compounds of magnesium, calcium, and other Group 2 elements. This often results in higher melting points and lower solubility for many calcium compounds when compared to similar compounds of sodium or potassium.
Common Compounds of Group 2 Elements
Alkaline earth metals form a wide array of important compounds that we encounter in daily life and industry. From simple oxides and hydrides to more complex salts like carbonates and sulfates, these compounds have diverse properties and uses. The common +2 oxidation state of the metals leads to predictable chemical formulas.
Some well-known examples include calcium carbonate (limestone) and various beryllium compounds used in specialised applications. Next, we’ll explore some of the most common types of compounds, including their formation and characteristics.
Hydrides, Oxides, and Hydroxides
Alkaline earth metals react with hydrogen to form hydrides. While elements like calcium do this directly, beryllium hydride must be prepared indirectly. These hydrides, such as calcium hydride, can react violently with water to produce hydrogen gas.
Oxides are formed when these metals react with oxygen. Calcium oxide, also known as quicklime, is a common example. These oxides can then react with water to form hydroxides. The basicity of these hydroxides increases as you go down the group.
Hydroxides like calcium hydroxide (slaked lime) and magnesium hydroxide (milk of magnesia) are widely used. While beryllium compounds like its hydroxide are amphoteric (acting as an acid or a base), the hydroxides of the heavier elements are distinctly basic.
Carbonates, Sulfates, and Nitrates
Carbonates, sulfates, and nitrates are three major classes of salts formed by alkaline earth metals. Calcium carbonate is incredibly common in nature, found as limestone, marble, and chalk. Other carbonates, like strontium carbonate, are also industrially important.
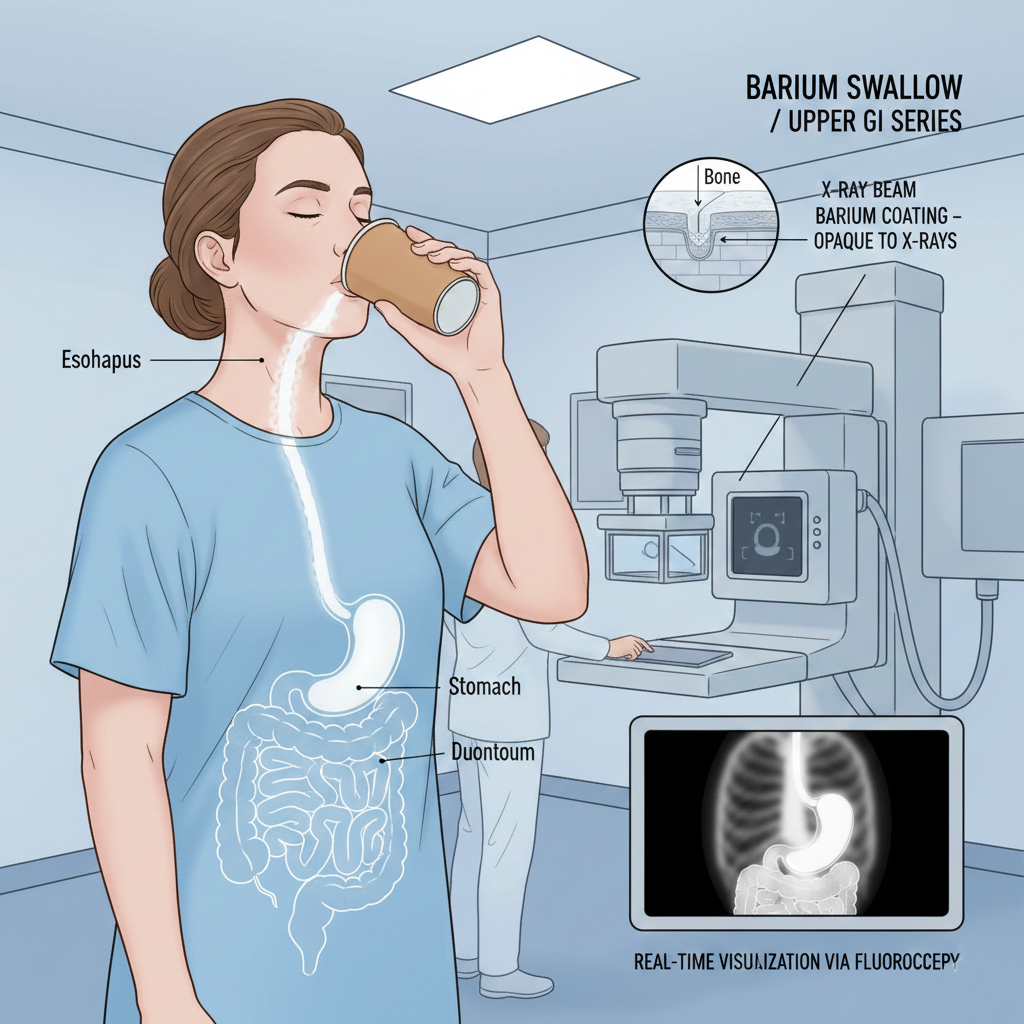
The sulfates of these metals show an interesting trend in solubility. Beryllium sulfate is quite soluble in water, but solubility decreases down the group, making barium sulfate nearly insoluble. This property makes barium sulfate ideal for medical X-rays of the digestive system, as it won’t be absorbed by the body. Calcium sulfate is better known as gypsum.
Nitrates of the alkaline earth metals are typically prepared by reacting their oxides or carbonates with nitric acid. Unlike the carbonates and sulfates, most nitrates are soluble in water. They are stable salts but will decompose upon heating.
Solubility Patterns Across the Group
The solubility of alkaline earth metal compounds in water follows some clear patterns, but a single rule doesn’t cover everything. The trend often depends on the negative ion involved. This is due to a delicate balance between the energy holding the crystal together and the energy released when the ions are hydrated by water.
For some compounds, solubility decreases as you move down the group. This is true for sulfates and carbonates. The smaller ions at the top of the group, like Be²⁺ and Mg²⁺, have a high charge density and are hydrated more easily, which promotes solubility.
Here is a summary of key solubility trends:
- Hydroxides: Solubility increases down the group.
- Sulfates: Solubility decreases down the group. BeSO₄ is soluble, while BaSO₄ is insoluble.
- Carbonates: Solubility decreases down the group.
- Halides (except fluorides): Solubility generally decreases down the group. The properties of many calcium salts, strontium compounds, and magnesium compounds are dictated by these solubility rules.
Everyday Uses and Applications in Industry
You might be surprised by how often you encounter alkaline earth metals and their compounds. From construction and metallurgy to medicine and consumer products, their applications are widespread. Their unique properties make them indispensable in many areas.
For example, magnesium alloys are used in the automotive industries to make lightweight parts, while calcium compounds are the backbone of the cement industry. Let’s explore some of their specific uses in metallurgy, medicine, and other industrial sectors.
Metallurgy and Manufacturing

In the field of metallurgy, alkaline earth metals are highly valued. Because they readily give up their electrons, elements like calcium and magnesium are used as a reducing agent to extract other metals from their ores. This process often involves high temperatures, where the alkaline earth metal removes oxygen from a metal oxide.
Calcium compounds are also essential in steelmaking, where they are used to remove impurities like sulfur and oxygen. Magnesium is famous for its use in strong, lightweight alloys. These alloys are crucial in the aerospace and automotive industries, where reducing weight improves fuel efficiency.
Barium, often sourced from the mineral barite, also has metallurgical applications. It is used in certain alloys and helps remove unwanted gases from vacuum tubes, a critical step in manufacturing electronics.
Medical and Biological Uses
The role of alkaline earth metals in living organisms is profound, particularly calcium. Calcium is a cornerstone of the human body, with calcium salts forming the primary structure of our bones and teeth. It is also essential for muscle function, nerve signaling, and blood clotting.
Magnesium is another vital element for life. It is present in every cell and is a critical component for over 300 enzymes in the body. It plays a key role in energy production and DNA synthesis.
Beyond their biological roles, some compounds have direct medical applications. Calcium carbonate is a common ingredient in antacids to neutralise stomach acid. In medical imaging, a patient might drink a solution of barium sulfate, which is opaque to X-rays, to get clear images of their digestive tract.
Other Industrial Applications
The uses of alkaline earth metals extend into many other industrial areas. Beryllium, for example, is almost transparent to X-rays. This property makes it perfect for making the “windows” in X-ray tubes and detectors. Beryllium compounds are also used in specialised alloys.
Strontium compounds are well-known for the brilliant red color they produce in fireworks and flares. Similarly, barium compounds produce a pale green color. These vibrant colors are created when the metal ions are heated.
Magnesium is highly flammable and burns with a very bright white light, making it a key ingredient in fireworks, flares, and incendiary devices. Other compounds like calcium sulfide have phosphorescent properties, meaning they can glow in the dark after being exposed to light. These diverse applications show just how versatile this group of elements is.

In conclusion, the alkaline earth metals represent an intriguing group in the Periodic Table with unique properties and significant applications. From their distinctive atomic structures to their reactivity trends, understanding these elements enhances our knowledge of chemistry and its practical implications. Whether you’re looking into their industrial applications or exploring their roles in medical sciences, the versatility of these metals is remarkable. Engaging with this topic not only deepens your appreciation for the building blocks of matter but also opens doors to various scientific fields.
The alkaline earth metals are the elements located in Group 2 of the Periodic Table. This family includes six elements: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and the radioactive element radium (Ra). They share similar chemical and physical properties due to their atomic structure.
The chemical reactivity of Group 2 elements increases as you move down the Periodic Table from beryllium to barium. This is because the atomic radius gets larger, making it easier for the atom to lose its two outer electrons and achieve a stable +2 oxidation state.
When handling alkaline earth metals, safety is crucial due to their chemical properties. The pure form of these metals can be highly reactive, especially with water and acids. Magnesium powder is flammable and burns at very high temperatures, so it requires careful handling. Protective gear should always be used.